Debabrata Biswas
National University of Singapore, Singapore
Title: Group A Streptococcal CXC chemokine-protease cleaves the human antimicrobial cathelicidin peptide LL-37 resulting in the loss of immunomodulatory functions of the peptide
Biography
Biography: Debabrata Biswas
Abstract
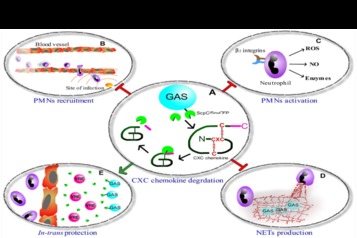
Statement of the Problem: Severe-soft tissue infections caused by group A streptococci (GAS) are characterized by a rapid dissemination of GAS followed by massive necrosis and tissue destruction. The human antimicrobial peptide, LL-37, is expressed during invasive GAS infections. It is believed that LL-37 antibacterial activity limits GAS spreading, since mice deficient of the LL-37 murine analog, CRAMP, is more sensitive than wild type mice to subcutaneous GAS challenge. LL-37 also directly recruits neutrophils to the site of infection and stimulates interleukin-8 (IL-8) production by keratinocytes. Thus, the immunomodulatory activity of LL-37 is aimed to exacerbate neutrophil response that is crucial for eradication of GAS from soft-tissue. Yet, analysis of debrided human soft-tissue samples revealed the coincidence of LL-37 along with viable GAS.
Theoretical Orientation: The GAS CXC-chemokine protease ScpC plays a central role in virulence through IL-8 cleavage preventing recruitment of neutrophils to the site of infection and reducing the production of neutrophil extracellular trap. Because of its immunomodulatory activities, we hypothesized that LL-37 may also serve as a substrate for ScpC. Functional significance of ScpC-mediated cleavage of LL-37 was studied in vitro and verified in vivo in the mouse model of human GAS soft-tissue infections.
Findings: Here, we demonstrate that immunomodulatory activity of LL-37 is crucial for controlling GAS spreading in soft-tissue. We found that GAS CXC-chemokine protease ScpC degrades and inactivates IL-8 as well as LL-37. ScpC cleaves the first 8 amino acids from the N-terminal of LL-37. This results in loss of its capacity to recruit neutrophil and stimulate IL-8 production by keratinocytes. In summary, the capacity of ScpC to shut down recruitment of neutrophils that is mediated through both IL-8 and LL-37 as well as inactivate LL-37-mediated IL-8 production by keratinocytes reflects a perfect adaptation of GAS to its human host.